Boiling Point Of Ethylene Glycol
Ethylene Glycol based water solutions are common in rut-transfer applications where the temperature in the heat transfer fluid tin can exist below 32oF (0oC). Ethylene glycol is also unremarkably used in heating applications that temporarily may not exist operated (cold) in surroundings with freezing conditions - such as cars and machines with water cooled engines.
Ethylene Glycol is the nigh common antifreeze fluid for standard heating and cooling applications. Ethylene glycol should be avoided if there is a slightest adventure of leakage to potable water or nutrient processing systems. Instead solutions based on propylene glycol are commonly used.
Specific oestrus, viscosity and specific weight of a water and ethylene glycol solution vary significantly with the percent of ethylene glycol and the temperature of the fluid. Properties differs so much from clean water that heat transfer systems with ethylene glycol should be calculated thoroughly for actual temperature and solution.
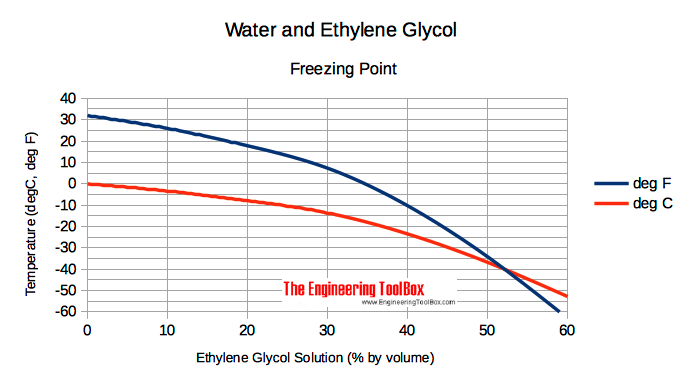
Freezing Betoken of Ethylene Glycol based Water Solutions
Freezing points of ethylene glycol based h2o solutions at various temperatures are indicated below
| Freezing Point | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethylene Glycol Solution (% by volume) | 0 | ten | 20 | 30 | 40 | l | lx | 80 | 90 | 100 | |
| Temperature | (oF) | 32 | 25.9 | 17.viii | 7.3 | -10.3 | -34.2 | -63 | ≈ -51 | ≈ -22 | nine |
| (oC) | 0 | -iii.4 | -vii.9 | -xiii.7 | -23.5 | -36.eight | -52.8 | ≈ -46 | ≈ -30 | -12.8 | |
- Propylene Glycol and Freezing Points
Due to possible slush creation, ethylene glycol and water solutions should not exist used in conditions close to freezing points.
Dynamic Viscosity of Ethylene Glycol based Water Solutions
Dynamic viscosity - μ - of ethylene glycol based water solutions at various temperatures are indicated below
| Dynamic Viscosity - μ - (centiPoise) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Temperature | Ethylene Glycol Solution (% by book) | |||||||
| (oF) | (oC) | 25 | 30 | xl | 50 | sixty | 65 | 100 |
| 0 | -17.viii | 1) | 1) | 15 | 22 | 35 | 45 | 310 |
| xl | iv.four | 3 | 3.v | 4.8 | half-dozen.5 | 9 | x.2 | 48 |
| 80 | 26.7 | 1.five | i.7 | two.2 | 2.eight | 3.viii | four.5 | 15.5 |
| 120 | 48.nine | 0.9 | one | i.3 | 1.v | 2 | ii.four | vii |
| 160 | 71.ane | 0.65 | 0.7 | 0.8 | 0.95 | 1.iii | ane.5 | 3.eight |
| 200 | 93.3 | 0.48 | 0.5 | 0.vi | 0.7 | 0.88 | 0.98 | 2.4 |
| 240 | 115.6 | 2) | 2) | 2) | 2) | 2) | 2) | one.eight |
| 280 | 137.8 | 2) | ii) | 2) | 2) | ii) | 2) | 1.2 |
- below freezing point
- higher up humid bespeak
Note! The dynamic viscosity of an ethylene glycol based water solution is increased compared with the dynamic viscosity of make clean water. Equally a consequence the head loss (pressure loss) in the a pipe organisation with ethylene glycol is increased compared to clean h2o.
Specific Gravity of Ethylene Glycol based Water Solutions
Specific gravity - SG - of ethylene glycol based water solutions at various temperatures are indicated below
| Specific Gravity- SG - | ||||||||
|---|---|---|---|---|---|---|---|---|
| Temperature | Ethylene Glycol Solution (% by book) | |||||||
| (oF) | (oC) | 25 | 30 | 40 | 50 | 60 | 65 | 100 |
| -40 | -40 | 1) | 1) | i) | 1) | i.12 | i.13 | 1) |
| 0 | -17.viii | 1) | one) | i.08 | 1.ten | 1.eleven | i.12 | 1.16 |
| forty | four.4 | 1.048 | i.057 | 1.07 | 1.088 | 1.i | 1.eleven | 1.145 |
| 80 | 26.vii | one.04 | one.048 | one.06 | 1.077 | 1.09 | 1.095 | 1.13 |
| 120 | 48.9 | one.03 | 1.038 | 1.05 | 1.064 | i.077 | 1.082 | 1.115 |
| 160 | 71.1 | 1.018 | 1.025 | i.038 | 1.05 | one.062 | 1.068 | one.i |
| 200 | 93.iii | 1.005 | 1.013 | 1.026 | 1.038 | i.049 | 1.054 | 1.084 |
| 240 | 115.6 | 2) | 2) | 2) | 2) | 2) | 2) | 1.067 |
| 280 | 137.8 | 2) | 2) | 2) | 2) | ii) | two) | 1.05 |
- below freezing point
- in a higher place boiling signal
Note! The specific gravity of ethylene glycol based water solutions are increased compared with specific gravity of clean water.
Densities of Ethylene Glycol based Water Solutions
Plough the screen to run across the whole table.
| Density - ρ - (kg/thousand3) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mass Fraction of Ethylene Glycol in Solution | Temperature - t - (oC) | |||||||||||
| -48 | -35 | -25 | -fourteen | -8 | -four | 0 | twenty | 40 | 60 | 80 | 100 | |
| 0 | thousand | 998 | 992 | 983 | 972 | 958 | ||||||
| 0.1 | 1019 | 1018 | 1014 | 1008 | 1000 | 992 | 984 | |||||
| 0.ii | 1038 | 1037 | 1036 | 1030 | 1022 | 1014 | 1005 | 995 | ||||
| 0.three | 1058 | 1056 | 1055 | 1054 | 1046 | 1037 | 1027 | 1017 | 1007 | |||
| 0.four | 1080 | 1077 | 1075 | 1073 | 1072 | 1063 | 1052 | 1041 | 1030 | 1018 | ||
| 0.5 | 1103 | 1100 | 1096 | 1093 | 1092 | 1090 | 1079 | 1067 | 1055 | 1042 | 1030 | |
| 0.6 | 1127 | 1124 | 1120 | 1115 | 1112 | 1110 | 1107 | 1095 | 1082 | 1068 | 1055 | 1042 |
- Online expansion book calculator
Case - Expansion Volume in a Heating System with Ethylene Glycol
A heating system with liquid volume 0.8 m3 is freeze protected with 50% (by mass, mass fraction 0.5) ethylene glycol. The installation temperature of the organization is downwardly to 0oC and the maximum medium operation temperature is 80oC.
From the table in a higher place nosotros see that the density of the solution at installation temperature can exist every bit high as 1090 kg/m3 - and the medium density at functioning temperature can be every bit low as 1042 kg/m3 .
The mass of the liquid at installation can be calculated as
minst = ρinst Fiveinst (one)
= (1090 kg/m3) (0.8 thousandiii)
= 872 kg
where
chiliadinst = mass of liquid at installation (kg)
ρinst = density at installation (kg/m3)
5 inst = liquid book at installation (yardiii)
Mass of the liquid in the system during operation will exist aforementioned equally the mass in system during installation
minst = mop (2)
= ρop 5op
where
thouop = mass of liquid at functioning (kg)
ρop = density atoperation (kg/g3)
V op = liquid book atoperation (m3)
(2) can exist modified to summate liquid operation volume as
5op = minst / ρop (2b)
= (872 kg) / (1042 kg/mthree )
= 0.837 one thousandthree
The required expansion book to avoid pressure tin be calculated every bit
ΔV = Vop - Vinst (three)
= (0.837 thousand3) - (0.8 m3)
= 0.037 k3
= 37 liter
where
ΔV = expansion volume (mthree)
Expansion volume tin be calculated equally
ΔV = ( ρinst / ρop - i) Vinst (4)
Specific Heat of Ethylene Glycol based Water Solutions
Specific Heat - cp - of ethylene glycol based water solutions at various temperatures are indicated beneath
Turn the screen to the whole table.
| Specific Heat - cp (Btu/lb oF) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethylene Glycol Solution (% by weight) | Temperature (°C) | |||||||||||||||
| -l | -40 | -30 | -xx | -ten | 0 | 10 | 20 | 30 | xl | fifty | 60 | seventy | fourscore | 90 | 100 | |
| 0 | 1.0038 | 1.0018 | 1.0004 | 0.99943 | 0.99902 | 0.99913 | 0.99978 | 1.0009 | 1.0026 | 1.0049 | 1.0076 | |||||
| 10 | 0.97236 | 0.97422 | 0.97619 | 0.97827 | 0.98047 | 0.98279 | 0.98521 | 0.98776 | 0.99041 | 0.99318 | 0.99607 | |||||
| 20 | 0.93576 | 0.93976 | 0.94375 | 0.94775 | 0.95175 | 0.95574 | 0.95974 | 0.96373 | 0.96773 | 0.97173 | 0.97572 | |||||
| 30 | 0.89373 | 0.89889 | 0.90405 | 0.90920 | 0.91436 | 0.91951 | 0.92467 | 0.92982 | 0.93498 | 0.94013 | 0.94529 | 0.95044 | ||||
| 40 | 0.84605 | 0.85232 | 0.85858 | 0.86484 | 0.87111 | 0.87737 | 0.88364 | 0.88990 | 0.89616 | 0.90243 | 0.90869 | 0.91496 | 0.92122 | |||
| l | 0.79288 | 0.80021 | 0.80753 | 0.81485 | 0.82217 | 0.82949 | 0.83682 | 0.84414 | 0.85146 | 0.85878 | 0.86610 | 0.87343 | 0.88075 | 0.88807 | ||
| 60 | 0.72603 | 0.73436 | 0.74269 | 0.75102 | 0.75935 | 0.76768 | 0.77601 | 0.78434 | 0.79267 | 0.80100 | 0.80933 | 0.81766 | 0.82599 | 0.83431 | 0.84264 | 0.85097 |
| 70 | 0.67064 | 0.67992 | 0.68921 | 0.69850 | 0.70778 | 0.71707 | 0.72636 | 0.73564 | 0.74493 | 0.75422 | 0.76350 | 0.77279 | 0.78207 | 0.79136 | 0.80065 | 0.80993 |
| 80 | 0.61208 | 0.62227 | 0.63246 | 0.64265 | 0.65285 | 0.66304 | 0.67323 | 0.68343 | 0.69362 | 0.70381 | 0.71401 | 0.72420 | 0.73439 | 0.74458 | 0.75478 | 0.76497 |
| ninety | 0.58347 | 0.59452 | 0.60557 | 0.61662 | 0.62767 | 0.63872 | 0.64977 | 0.66082 | 0.67186 | 0.68291 | 0.69396 | 0.70501 | 0.71606 | |||
| 100 | 0.53282 | 0.54467 | 0.55652 | 0.56838 | 0.58023 | 0.59209 | 0.60394 | 0.61579 | 0.62765 | 0.63950 | 0.65136 | 0.66321 | ||||
- Freezing indicate 100% ethylene glycol at atmospheric pressure is -12.8oC (9oF)
- 1 Btu/(lbthousand oF) = 4,186.8 J/(kg Grand) = i kcal/(kgoC)
Note! The specific estrus of ethylene glycol based water solutions are less than the specific estrus of make clean water. For a rut transfer organisation with ethylene glycol the circulated volume must be increased compared to a system but with water.
In a 50% solution with operational temperatures above 36 oF the specific rut chapters is decreased with approximately 20%. The reduced heat chapters must exist compensated by circulating more fluid.
Note! The density of ethylene glycol is higher than water - check the specific gravity (SG) table above, so the internet impact on the oestrus transport capacity is reduced. Case - the specific rut of an ethylene glycol water solution 50% / 50% is 0.815 at 80 oF (26.seven oC). Specific gravity at the same weather is 1.077. The internet impact tin be estimated to 0.815 * 1.077 = 0.877.
Machine antifreeze solutions should non be used in HVAC systems considering they contain silicates that may cause fouling. Silicates in automobile antifreeze are used to protect aluminum engine parts.
Note! Distilled or deionized h2o should be used for ethylene glycol solutions. City water may be treated with chlorine which is corrosive.
Systems for automatic makeup water should non be used since a leakage would contaminate the environs and dilute the antifreeze protection of the organization.
Boiling Points Ethylene Glycol Solutions
For total table with Boiling Points - rotate the screen!
| Boiling Signal | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethylene Glycol Solution (% by volume) | 0 | 10 | xx | thirty | xl | 50 | 60 | 70 | lxxx | 90 | 100 | |
| Temperature | (oF) | 212 | 214 | 216 | 220 | 220 | 225 | 232 | 245 | 260 | 288 | 386 |
| (oC) | 100 | 101.1 | 102.2 | 104.4 | 104.4 | 107.2 | 111.ane | 118 | 127 | 142 | 197 | |
Increase in Flow required for a fifty% Ethylene Glycol Solution
Increment in circulated flow for 50% ethylene glycol solutions compared with clean water are indicated in the table below
| Fluid Temperature | Flow Increase (%) | |
|---|---|---|
| (oF) | (oC) | |
| 40 | four.4 | 22 |
| 100 | 37.8 | 16 |
| 140 | sixty.0 | xv |
| 180 | 82.2 | 14 |
| 220 | 104.4 | 14 |
Pressure Drib Correction and Combined Pressure Drop and Volume Catamenia Correction for 50% Ethylene Glycol Solution
Pressure drop correction and combined pressure drib and catamenia increment correction for 50% ethylene glycol solutions compared with clean h2o are indicated in the table below
| Fluid Temperature | Pressure Drop Correction with Flow Rates Equal (%) | Combined Pressure Drop and Period Rate Correction (%) | |
|---|---|---|---|
| (oF) | (oC) | ||
| 40 | 4.4 | 45 | 114 |
| 100 | 37.eight | ten | 49 |
| 140 | sixty.0 | 0 | 32 |
| 180 | 82.2 | -half dozen | 23 |
| 220 | 104.4 | -ten | 18 |
Boiling Point Of Ethylene Glycol,
Source: https://www.engineeringtoolbox.com/ethylene-glycol-d_146.html
Posted by: carterintim1962.blogspot.com


0 Response to "Boiling Point Of Ethylene Glycol"
Post a Comment